Dr. Happer’s Advice From Global Warming Dialogue
There’s renewed interest in this interchange between William Happer and David Karoly conducted by The Best Schools in their Civil Global Warming Dialogue. Excerpts below are from William Happer’s Major Statement, which is no longer available. Instead, there is an extensive William Happer Interview on Global Warming from September 7, 2021. The David Karoly Interview is available from Andy May’s website.
William Happer’s Major Statement at the Best Schools Global Warming Dialogue is CO₂ will be a major benefit to the Earth.
Some people claim that increased levels of atmospheric CO2 will cause catastrophic global warming, flooding from rising oceans, spreading tropical diseases, ocean acidification, and other horrors. But these frightening scenarios have almost no basis in genuine science. This Statement reviews facts that have persuaded me that more CO2 will be a major benefit to the Earth.
Discussions of climate today almost always involve fossil fuels. Some people claim that fossil fuels are inherently evil. Quite the contrary, the use of fossil fuels to power modern society gives the average person a standard of living that only the wealthiest could enjoy a few centuries ago. But fossil fuels must be extracted responsibly, minimizing environmental damage from mining and drilling operations, and with due consideration of costs and benefits. Similarly, fossil fuels must be burned responsibly, deploying cost-effective technologies that minimize emissions of real pollutants such as fly ash, carbon monoxide, oxides of sulfur and nitrogen, heavy metals, volatile organic compounds, etc.
Extremists have conflated these genuine environmental concerns with the emission of CO2, which cannot be economically removed from exhaust gases. Calling CO2 a “pollutant” that must be eliminated, with even more zeal than real pollutants, is Orwellian Newspeak.[3] “Buying insurance” against potential climate disasters by forcibly curtailing the use of fossil fuels is like buying “protection” from the mafia. There is nothing to insure against, except the threats of an increasingly totalitarian coalition of politicians, government bureaucrats, crony capitalists, thuggish nongovernmental organizations like Greenpeace, etc.
Figure 1. The ratio, RCO2, of past atmospheric CO2 concentrations to average values (about 300 ppm) of the past few million years, This particular proxy record comes from analyzing the fraction of the rare stable isotope 13C to the dominant isotope 12C in carbonate sediments and paleosols. Other proxies give qualitatively similar results.[
Life on Earth does better with more CO2. CO2 levels are increasing
Fig. 1 summarizes the most important theme of this discussion. It is not true that releasing more CO2 into the atmosphere is a dangerous, unprecedented experiment. The Earth has already “experimented” with much higher CO2 levels than we have today or that can be produced by the combustion of all economically recoverable fossil fuels.
Radiative cooling of the Earth and The Role of Water and Clouds
Without sunlight and only internal heat to keep warm, the Earth’s absolute surface temperature T would be very cold indeed. A first estimate can be made with the celebrated Stefan-Boltzmann formula
J= εσT^4 [Equation 1 ]
where J is the thermal radiation flux per unit of surface area, and the Stefan-Boltzmann constant (originally determined from experimental measurements) has the value σ = 5.67 × 10-8 W/(m2K4). If we use this equation to calculate how warm the surface would have to be to radiate the same thermal energy as the mean solar flux, Js = F/4 = 340 W/m2, we find Ts = 278 K or 5 C, a bit colder than the average temperature (287 K or 14 C) of the Earth’s surface,[19] but “in the ball park.”
Figure 5. The temperature profile of the Earth’s atmosphere.[20] This illustration is for mid-latitudes, like Princeton, NJ, at 40.4o N, where the tropopause is usually at an altitude of about 11 km. The tropopause is closer to 17 km near the equator, and as low as 9 km near the north and south poles.
These estimates can be refined by taking into account the Earth’s atmosphere. In the Interview we already discussed the representative temperature profile, Fig. 5. The famous “blue marble” photograph of the Earth,[21] reproduced in Fig. 6, is also very instructive. Much of the Earth is covered with clouds, which reflect about 30% of sunlight back into space, thereby preventing its absorption and conversion to heat. Rayleigh scattering (which gives the blue color of the daytime sky) also deflects shorter-wavelength sunlight back to space and prevents heating.
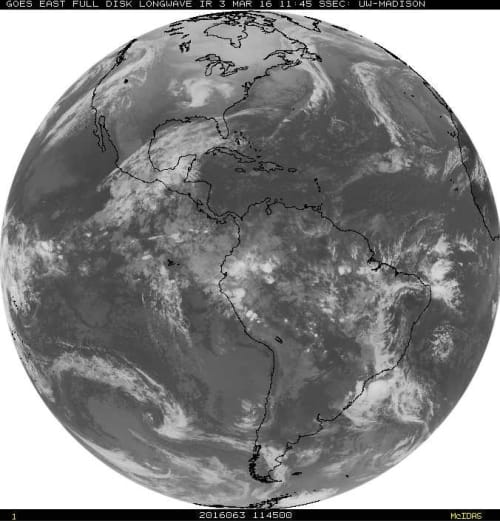
Today, whole-Earth images analogous to Fig. 6 are continuously recorded by geostationary satellites, orbiting at the same angular velocity as the Earth, and therefore hovering over nearly the same spot on the equator at an altitude of about 35,800 km.[23] In addition to visible images, which can only be recorded in daytime, the geostationary satellites record images of the thermal radiation emitted both day and night.
Figure 7. Radiation with wavelengths close to the 10.7 µ (1µ = 10-6m), as observed with a geostationary satellite over the western hemisphere of the Earth.[23] This is radiation in the infrared window of Fig. 4, where the surface can radiate directly to space from cloud-free regions.
Fig. 7 shows radiation with wavelengths close to 10.7 µ in the “infrared window” of the absorption spectrum shown in Fig. 4, where there is little absorption from either the main greenhouse gas, H2O, or from less-important CO2. Darker tones in Fig. 7 indicate more intense radiation. The cold “white” cloud tops emit much less radiation than the surface, which is “visible” at cloud-free regions of the Earth. This is the opposite from Fig. 6, where maximum reflected sunlight is coming from the white cloud tops, and much less reflection from the land and ocean, where much of the solar radiation is absorbed and converted to heat.
As one can surmise from Fig. 6 and Fig. 7, clouds are one of the most potent factors that control the surface temperature of the earth. Their effects are comparable to those of the greenhouse gases, H2O and CO2, but it is much harder to model the effects of clouds. Clouds tend to cool the Earth by scattering visible and near-visible solar radiation back to space before the radiation can be absorbed and converted to heat. But clouds also prevent the warm surface from radiating directly to space. Instead, the radiation comes from the cloud tops that are normally cooler than the surface. Low-cloud tops are not much cooler than the surface, so low clouds are net coolers. In Fig. 7, a large area of low clouds can be seen off the coast of Chile. They are only slightly cooler than the surrounding waters of the Pacific Ocean in cloud-free areas.
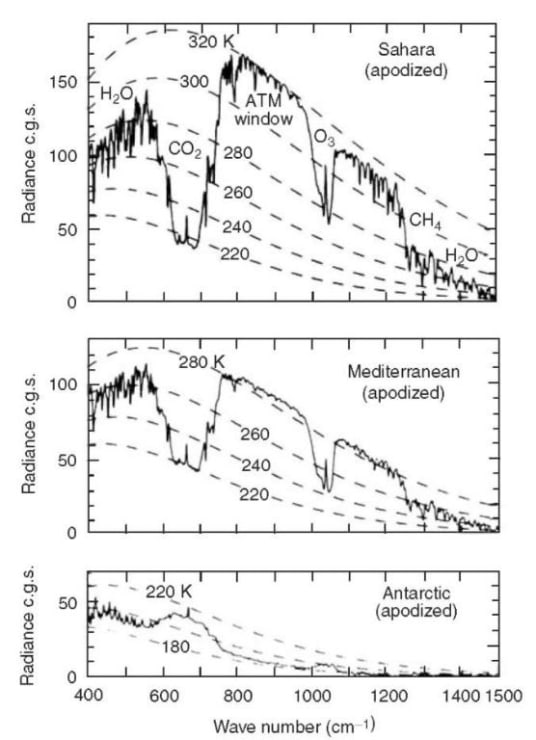
Figure 8. Spectrally resolved, vertical upwelling thermal radiation I from the Earth, the jagged lines, as observed by a satellite.[28] The smooth, dashed lines are theoretical Planck brightnesses, B, for various temperatures. The vertical units are 1 c.g.s = 1 erg/(s cm2 sr cm-1) = 1 mW/(m2 sr cm-1).
Except at the South Pole, the data of Fig. 8 show that the observed thermal radiation from the Earth is less intense than Planck radiation from the surface would be without greenhouse gases. Although the surface radiation is completely blocked in the bands of the greenhouse gases, as one would expect from Fig. 4, radiation from H2O and CO2 molecules at higher, colder altitudes can escape to space. At the “emission altitude,” which depends on frequency ν, there are not enough greenhouse molecules left overhead to block the escape of radiation. The thermal emission cross section of CO2 molecules at band center is so large that the few molecules in the relatively warm upper stratosphere (see Fig. 5) produce the sharp spikes in the center of the bands of Fig. 8. The flat bottoms of the CO2 bands of Fig 8 are emission from the nearly isothermal lower stratosphere (see Fig. 5) which has a temperature close to 220 K over most of the Earth.
It is hard for H2O molecules to reach cold, higher altitudes, since the molecules condense onto snowflakes or rain drops in clouds. So the H2O emissions to space come from the relatively warm and humid troposphere, and they are only moderately less intense than the Planck brightness of the surface. CO2 molecules radiate to space from the relatively dry and cold lower stratosphere. So for most latitudes, the CO2 band observed from space has much less intensity than the Planck brightness of the surface.
Concentrations of H2O vapor can be quite different at different locations on Earth. A good example is the bottom panel of Fig. 8, the thermal radiation from the Antarctic ice sheet, where almost no H2O emission can be seen. There, most of the water vapor has been frozen onto the ice cap, at a temperature of around 190 K. Near both the north and south poles there is a dramatic wintertime inversion[30] of the normal temperature profile of Fig. 5. The ice surface becomes much colder than most of the troposphere and lower stratosphere.
Cloud tops in the intertropical convergence zone (ITCZ) can reach the tropopause and can be almost as cold as the Antarctic ice sheet. The spectral distribution of cloud-top radiation from the ITCZ looks very similar to cloud-free radiation from the Antarctic ice, shown on the bottom panel of Fig. 8.
Convection
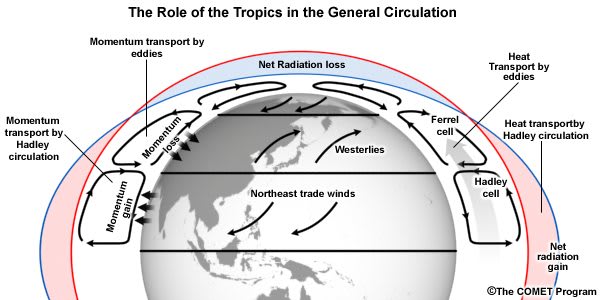
Radiation, which we have discussed above, is an important part of the energy transfer budget of the earth, but not the only part. More solar energy is absorbed in the tropics, near the equator, where the sun beats down nearly vertically at noon, than at the poles where the noontime sun is low on the horizon, even at midsummer, and where there is no sunlight at all in the winter. As a result, more visible and near infrared solar radiation (“short-wave radiation” or SWR) is absorbed in the tropics than is radiated back to space as thermal radiation (“long-wave radiation” or LWR). The opposite situation prevails near the poles, where thermal radiation releases more energy to space than is received by sunlight. Energy is conserved because the excess solar energy from the tropics is carried to the poles by warm air currents, and to a lesser extent, by warm ocean currents. The basic physics is sketched in Fig. 11.[35]
Figure 11. Most sunlight is absorbed in the tropics, and some of the heat energy is carried by air currents to the polar regions to be released back into space as thermal radiation. Along with energy, angular momentum — imparted to the air from the rotating Earth’s surface near the equator — is transported to higher northern and southern latitudes, where it is reabsorbed by the Earth’s surface. The Hadley circulation near the equator is largely driven by buoyant forces on warm, solar-heated air, but for mid latitudes the “Coriolis force” due to the rotation of the earth leads to transport of energy and angular momentum through slanted “baroclinic eddies.” Among other consequences of the conservation of angular momentum are the easterly trade winds near the equator and the westerly winds at mid latitudes.
Equilibrium Climate Sensitivity
If increasing CO2 causes very large warming, harm can indeed be done. But most studies suggest that warmings of up to 2 K will be good for the planet,[38] extending growing seasons, cutting winter heating bills, etc. We will denote temperature differences in Kelvin (K) since they are exactly the same as differences in Celsius (C). A temperature change of 1 K = 1 C is equal to a change of 1.8 Fahrenheit (F).
If a 50% increase of CO2 were to increase the temperature by 3.4 K, as in Arrhenius’s original estimate mentioned above, the doubling sensitivity would be S = 3.4 K/log2(1.5) = 5.8 K. Ten years later, on page 53 of his popular book, Worlds in the Making: The Evolution of the Universe,[40] Arrhenius again states the logarithmic law of warming, with a slightly smaller climate sensitivity, S = 4 K.
Convection of the atmosphere, water vapor, and clouds all interact in a complicated way with the change of CO2 to give the numerical value of the doubling sensitivity S of Eq. (21). Remarkably, Arrhenius somehow guessed the logarithmic dependence on CO2 concentration before Planck’s discovery of how thermal radiation really works.
More than a century after Arrhenius, and after the expenditure of many tens of billions of dollars on climate science, the official value of S still differs little from the guess that Arrhenius made in 1912: S = 4 K.
Could it be that the climate establishment does not want to work itself out of a job?

Overestimate of Sensitivity
Contrary to the predictions of most climate models, there has been very little warming of the Earth’s surface over the last two decades. The discrepancy between models and observations issummarized by Fyfe, Gillett, and Zwiers, as shown in the Fyfe Fig.1 above.
At this writing, more than 50 mechanisms have been proposed to explain the discrepancy of Fyfe Fig.1. These range from aerosol cooling to heat absorption by the ocean. Some of the more popular excuses for the discrepancy have been summarized by Fyfe, et al. But the most straightforward explanation for the discrepancy between observations and models is that the doubling sensitivity, which most models assume to be close to the “most likely” IPCC value, S = 3 K, is much too large.
If one assumes negligible feedback, where other properties of the atmosphere change little in response to additions of CO2, the doubling efficiency can be estimated to be about S = 1 K, for example, as we discussed in connection with Eq. (19). The much larger doubling sensitivities claimed by the IPCC, which look increasingly dubious with each passing year, are due to “positive feedbacks.” A favorite positive feedback is the assumption that water vapor will be lofted to higher, colder altitudes by the addition of more CO2, thereby increasing the effective opacity of the vapor. Changes in cloudiness can also provide either positive feedback which increases S or negative feedback which decreases S. The simplest interpretation of the discrepancy of Fig. 13 and Fig. 14 is that the net feedback is small and possibly even negative. Recent work by Harde indicates a doubling sensitivity of S = 0.6 K.[46]

Figure 17. The analysis of satellite observations by Dr. Randall J. Donohohue and co-workers[53] shows a clear greening of the earth from the modest increase of CO2 concentrations from about 340 ppm to 400 ppm from the year 1982 to 2010. The greening is most pronounced in arid areas where increased CO2 levels diminish the water requirement of plants.
Benefits of CO2
More CO2 in the atmosphere will be good for life on planet earth. Few realize that the world has been in a CO2 famine for millions of years — a long time for us, but a passing moment in geological history. Over the past 550 million years since the Cambrian, when abundant fossils first appeared in the sedimentary record, CO2 levels have averaged many thousands of parts per million (ppm), not today’s few hundred ppm, which is not that far above the minimum level, around 150 ppm, when many plants die of CO2 starvation.
All green plants grow faster with more atmospheric CO2. It is found that the growth rate is approximately proportional to the square root of the CO2 concentrations, so the increase in CO2 concentrations from about 300 ppm to 400 ppm over the past century should have increased growth rates by a factor of about √(4/3) = 1.15, or 15%. Most crop yields have increased by much more than 15% over the past century. Better crop varieties, better use of fertilizer, better water management, etc., have all contributed. But the fact remains that a substantial part of the increase is due to more atmospheric CO2.
But the nutritional value of additional CO2 is only part of its benefit to plants. Of equal or greater importance, more CO2 in the atmosphere makes plants more drought-resistant. Plant leaves are perforated by stomata, little holes in the gas-tight surface skin that allow CO2 molecules to diffuse from the outside atmosphere into the moist interior of the leaf where they are photosynthesized into carbohydrates.
In the course of evolution, land plants have developed finely tuned feedback mechanisms that allow them to grow leaves with more stomata in air that is poor in CO2, like today, or with fewer stomata for air that is richer in CO2, as has been the case over most of the geological history of land plants.[51] If the amount of CO2 doubles in the atmosphere, plants reduce the number of stomata in newly grown leaves by about a factor of two. With half as many stomata to leak water vapor, plants need about half as much water. Satellite observations like those of Fig. 17 from R.J. Donohue, et al.,[52] have shown a very pronounced “greening” of the Earth as plants have responded to the modest increase of CO2 from about 340 ppm to 400 ppm during the satellite era. More greening and greater agricultural yields can be expected as CO2 concentrations increase further.
Climate Science
Droughts, floods, heat waves, cold snaps, hurricanes, tornadoes, blizzards, and other weather- and climate-related events will complicate our life on Earth, no matter how many laws governments pass to “stop climate change.” But if we understand these phenomena, and are able to predict them, they will be much less damaging to human society. So I strongly support high-quality research on climate and related fields like oceanography, geology, solar physics, etc. Especially important are good measurement programs like the various satellite measurements of atmospheric temperature[59] or the Argo[60] system of floating buoys that is revolutionizing our understanding of ocean currents, temperature, salinity, and other important properties.
But too much “climate research” money is pouring into very questionable efforts, like mitigation of the made-up horrors mentioned above. It reminds me of Gresham’s Law: “Bad money drives out good.”[61] The torrent of money showered on scientists willing to provide rationales, however shoddy, for the war on fossil fuels, and cockamamie mitigation schemes for non-existent problems, has left insufficient funding for honest climate science.
Summary
The Earth is in no danger from increasing levels of CO2. More CO2 will be a major benefit to the biosphere and to humanity. Some of the reasons are:
- As shown in Fig. 1, much higher CO2 levels than today’s prevailed over most last 550 million years of higher life forms on Earth. Geological history shows that the biosphere does better with more CO2.
- As shown in Fig. 13 and Fig. 14, observations over the past two decades show that the warming predicted by climate models has been greatly exaggerated. The temperature increase for doubling CO2 levels appears to be close to the feedback-free doubling sensitivity of S =1 K, and much less than the “most likely” value S = 3 K promoted by the IPCC and assumed in most climate models.
- As shown in Fig. 12, if CO2 emissions continue at levels comparable to those today, centuries will be needed for the added CO2 to warm the Earth’s surface by 2 K, generally considered to be a safe and even beneficial amount.
- Over the past tens of millions of years, the Earth has been in a CO2 famine with respect to the optimal levels for plants, the levels that have prevailed over most of the geological history of land plants. There was probably CO2 starvation of some plants during the coldest periods of recent ice ages. As shown in Fig. 15–17, more atmospheric CO2 will substantially increase plant growth rates and drought resistance.
There is no reason to limit the use of fossil fuels because they release CO2 to the atmosphere. However, fossil fuels do need to be mined, transported, and burned with cost-effective controls of real environmental problems — for example, fly ash, oxides of sulfur and nitrogen, volatile organic compounds, groundwater contamination, etc.
Sometime in the future, perhaps by the year 2050 when most of the original climate crusaders will have passed away, historians will write learned papers on how it was possible for a seemingly enlightened civilization of the early 21st century to demonize CO2, much as the most “Godly” members of society executed unfortunate “witches” in earlier centuries.
Dr. William Happer Background: Co-Founder and current Director of the CO2 Coalition, Dr. William Happer, Professor Emeritus in the Department of Physics at Princeton University, is a specialist in modern optics, optical and radiofrequency spectroscopy of atoms and molecules, radiation propagation in the atmosphere, and spin-polarized atoms and nuclei.
From September 2018 to September 2019, Dr. Happer served as Deputy Assistant to the President and Senior Director of Emerging Technologies on the National Security Council.
He has published over 200 peer-reviewed scientific papers. He is a Fellow of the American Physical Society, the American Association for the Advancement of Science, and a member of the American Academy of Arts and Sciences, the National Academy of Sciences and the American Philosophical Society. He was awarded an Alfred P. Sloan Fellowship in 1966, an Alexander von Humboldt Award in 1976, the 1997 Broida Prize and the 1999 Davisson-Germer Prize of the American Physical Society, and the Thomas Alva Edison Patent Award in 2000.
This commentary was originally posted on the iowaclimate.org website. To read more click here.